The quality and consistency of pre-analytical processes are crucial for data integrity, ultimately determining the success of clinical trials and longitudinal studies. Given the significant time and financial investments in these studies1,2, maintaining high-quality and consistent samples is essential to avoid compromised sample integrity, resulting in inaccurate data and invalid or inconclusive study outcomes. However, inconsistencies in the pre-analytical phase are not uncommon; reports have held pre-analytical variables responsible for up to 75% of laboratory errors3,4. This sheds light on the need for rigorous processes to guarantee that sample integrity is maintained throughout sample collection, transportation, processing, and storage. Here, we will discuss the key components of robust pre-analytical work, explore common challenges, and suggest strategies to ensure data integrity and validity across clinical, longitudinal, and research studies.

Key Considerations for Robust Pre-analytical Work
- Sample Collection and Processing: Proper sample preparation is the first step in ensuring sample integrity and analytical accuracy. Common sample collection and processing challenges include suboptimal collection apparatus, processing delays, inappropriate processing temperatures, and human errors5. These issues, which may seem minor, can have significant impacts: damaging proteins, DNA, and RNA, affecting analytical outcomes, and making distinguishing between true biological changes and procedural artifacts challenging6. Moreover, there is often a lack of standardization in pre-analytical processes, which can lead to variable results, even among case-matched samples7. Implementing strict functional quality control (FQC) measures, including visual inspection and functional QC, is critical to retaining sample quality, avoiding wasted time, resources, and money, and ascertaining the validity of research findings8.
- Sample Handling and Storage: [2] After collection and processing, samples must be aliquoted and stored under specific, temperature-controlled conditions. The optimal storage temperature is determined by the sample type, storage duration, and retrieval frequency, and getting this right is pivotal since incorrect storage temperatures can lead to loss of sample viability and integrity, particularly detrimental for precious patient samples or rare cell lines. Selecting the most suitable storage vessel and strategically storing aliquots are also essential for preserving sample utility and ensuring efficient tracking and retrieval9. Disaster recovery measures, such as backup generators, alarmed continuous temperature monitoring systems, and on-call technicians, are critical to protect against catastrophic sample loss in case of system failure or natural disaster. Moreover, implementing measures to prevent sample degradation and contamination during handling, such as limiting freeze-thaw cycles and handling samples under aseptic environments, is essential for preserving sample quality and ensuring the reliability of subsequent analyses10–12.
- Analytical Considerations: The strategic selection of the most optimal analytical method considering the impact of pre-analytical processes is an often overlooked but nonetheless critical component of the pre-analytical phase, with significant implications for the quality and consistency of research outcomes. When planning projects, researchers should consider factors related to pre-analytical processes, including the sensitivity of assays to sample degradation and the requirement for fresh samples13, alongside practical limitations such as resource availability and processing capacity. Distinguishing actual biological changes from pre-analytical variations is essential for ensuring data validity and facilitating comparisons across studies14. This requires rigorous pre-analytical QC and detailed documentation of sample handling procedures to identify and minimize pre-analytical errors, ultimately conserving resources by avoiding the analysis of degraded or unsuitable samples. Moreover, variability in equipment, methodologies, and processing techniques between different sites can introduce notable inconsistencies in data and should be standardized or minimized by using a single service provider15.
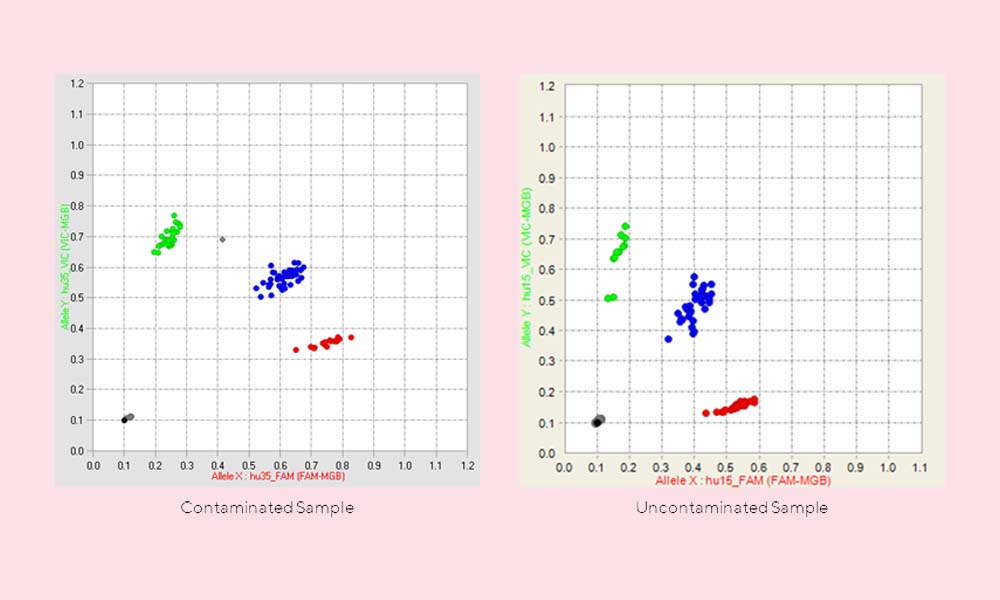
The Global Integrated Analytical Biorepository Strategy for Robust Pre-analytical Work
Sampled is the world’s first Global Integrated Analytical Biorepository (GIAB)[3] , providing a new, innovative pathway for biorepository services by combining traditional sample storage with a comprehensive array of pre-analytical and analytical services. Unlike standard biorepositories that focus mainly on sample storage and access, Sampled offers an integrated solution encompassing sample storage, processing, and logistics, cellular services, advanced multiomics, and bioinformatic analysis. This integrated approach supports a wide range of research needs through streamlined sample management, analysis, and reporting, thus accelerating research and development efforts. Notably, the integration of all the required services of a research project, from sample collection to data analysis and transfer, along with a full chain of custody and robust pre-analytical measures, ensures sample integrity and data validity throughout the process.
Sampled’s GIAB supports robust pre-analytical work through services such as:
- Clinical and research kitting
- Robust sample accessioning procedures
- Stringent QC
- Disaster recovery
- Transparent reporting
Ultimately, the GIAB approach ensures sample integrity and data reliability by coupling streamlined sample collection support with stringent quality controls, advanced sample accessioning, and a customizable reporting system. Its integrated, end-to-end model facilitates seamless sample collection to data reporting by a single provider, guaranteeing accurate and reliable data for successful research and clinical analysis and representing a comprehensive solution to the significant challenges associated with suboptimal pre-analytical work.
If you’re interested in learning more about the key components of robust pre-analytical work, check out our latest white paper. We delve deeper into the specifics and explore how Sampled’s GIAB approach supports this critical research phase for data you can trust.
References
1. Wouters OJ, McKee M, Luyten J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA. 2020;323(9):844. doi:10.1001/jama.2020.1166
2. Brown DG, Wobst HJ, Kapoor A, Kenna LA, Southall N. Clinical development times for innovative drugs. Nat Rev Drug Discov. 2022;21(11):793-794. doi:10.1038/d41573-021-00190-9
3. Plebani M. Quality indicators to detect pre-analytical errors in laboratory testing. Clin Biochem Rev. 2012;33(3):85-88.
4. Naz S, Mumtaz A, Sadaruddin A. Preanalytical Errors and their Impact on Tests in Clinical Laboratory Practice. Pak J Med Res. Published online 2012.
5. Agrawal L, Engel KB, Greytak SR, Moore HM. Understanding preanalytical variables and their effects on clinical biomarkers of oncology and immunotherapy. Semin Cancer Biol. 2018;52:26-38. doi:10.1016/j.semcancer.2017.12.008
6. Holland NT, Smith MT, Eskenazi B, Bastaki M. Biological sample collection and processing for molecular epidemiological studies. Mutat Res Mutat Res. 2003;543(3):217-234. doi:10.1016/S1383-5742(02)00090-X
7. Masucci GV, Cesano A, Hawtin R, et al. Validation of biomarkers to predict response to immunotherapy in cancer: Volume I — pre-analytical and analytical validation. J Immunother Cancer. 2016;4(1):76. doi:10.1186/s40425-016-0178-1
8. Ntzifa A, Lianidou E. Pre-analytical conditions and implementation of quality control steps in liquid biopsy analysis. Crit Rev Clin Lab Sci. 2023;60(8):573-594. doi:10.1080/10408363.2023.2230290
9. ISBER. Best Practices: Recommendations for Repositories Fifth Edition. Published online 2023. Accessed January 29, 2024. https://cdn.ymaws.com/www.isber.org/resource/resmgr/best_practices/ISBERBestPractices-5thEditio.pdf
10. Comstock GW, Burke AE, Norkus EP, Gordon GB, Hoffman SC, Helzlsouer KJ. Effects of Repeated Freeze-Thaw Cycles on Concentrations of Cholesterol, Micronutrients, and Hormones in Human Plasma and Serum. Am J Epidemiol. 2008;168(7):827-830. doi:10.1093/aje/kwn327
11. Permenter J, Ishwar A, Rounsavall A, et al. Quantitative analysis of genomic DNA degradation in whole blood under various storage conditions for molecular diagnostic testing. Mol Cell Probes. 2015;29(6):449-453. doi:10.1016/j.mcp.2015.07.002
12. Logie JJ, Chaloner C. A national survey of specimen contamination in the UK. Ann Clin Biochem Int J Lab Med. 2019;56(2):219-227. doi:10.1177/0004563218812500
13. Magnette A, Chatelain M, Chatelain B, Ten Cate H, Mullier F. Pre-analytical issues in the haemostasis laboratory: guidance for the clinical laboratories. Thromb J. 2016;14(1):49. doi:10.1186/s12959-016-0123-z
14. Monach PA. Repeating tests: different roles in research studies and clinical medicine. Biomark Med. 2012;6(5):691-703. doi:10.2217/bmm.12.57
15. Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non–Small Cell Lung Cancer. JAMA Oncol. 2017;3(8):1051. doi:10.1001/jamaoncol.2017.0013

