In the fast-paced world of modern biomedical laboratories, efficiency is key. That also applies to sample accessioning – the process of receiving, documenting, and preparing samples for testing.
Historically, many labs have relied on manual accessioning methods, which can be time-consuming and error-prone. Today, digital manifests are transforming how laboratories manage samples, offering significant time savings and reducing the risk of errors.
Here, we will explore the challenges associated with manual accessioning and how switching to digital processes can streamline the process by improving efficiency and accuracy.
The Challenges of Manual Accessioning
Manual accessioning involves physically labeling samples, manually entering data into logs, and tracking sample status through manually updated records. While simple, this process is inherently slow and labor-intensive. Laboratory staff must individually handle, label, and document each sample, which not only takes time but also introduces multiple opportunities for human error.
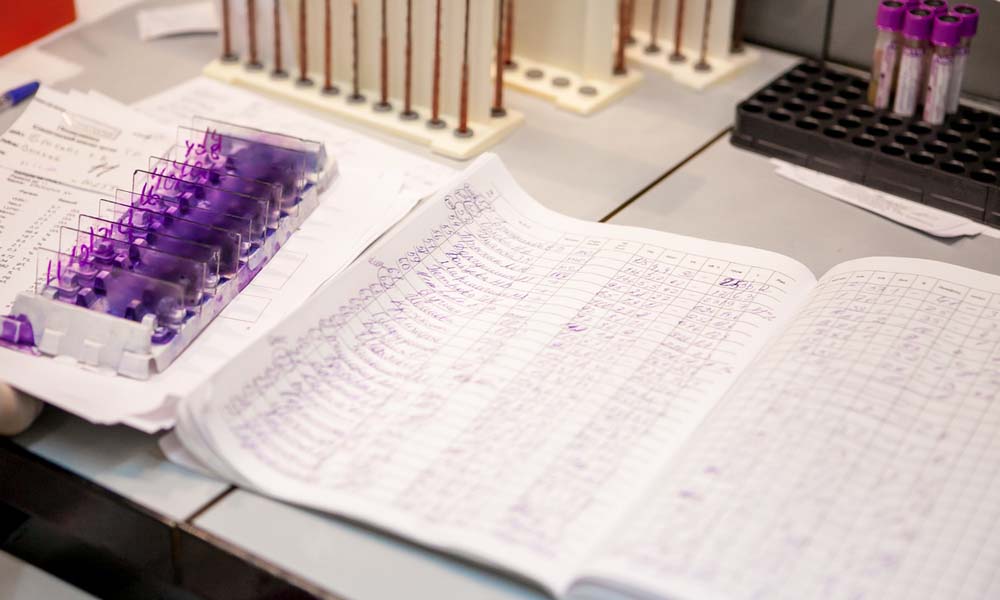
Common errors associated with manual processes include sample mislabeling, transcription mistakes, and incomplete documentation1–3. These errors can lead to serious consequences, such as incorrect test results, delays in processing, and compromised data integrity. In clinical settings, such errors can impact patient safety, leading to misdiagnosis or delayed diagnosis. In research settings, data errors can invalidate results, wasting valuable time and resources.
Moreover, as sample volumes increase, these issues become more pronounced. Labs may struggle to keep up with the workload, leading to backlogs that further slow the testing process. During high-demand periods, the limitations of manual accessioning have become particularly evident, underscoring the need for more efficient solutions4.
How Digital Manifests Improve Efficiency
Digital manifests offer a powerful alternative to manual accessioning by streamlining the entire process through automation. By using technologies like barcodes and radio-frequency identification (RFID) tags, digital systems can automatically capture and store sample information, eliminating the need for manual data entry and reducing the risk of errors2,5.
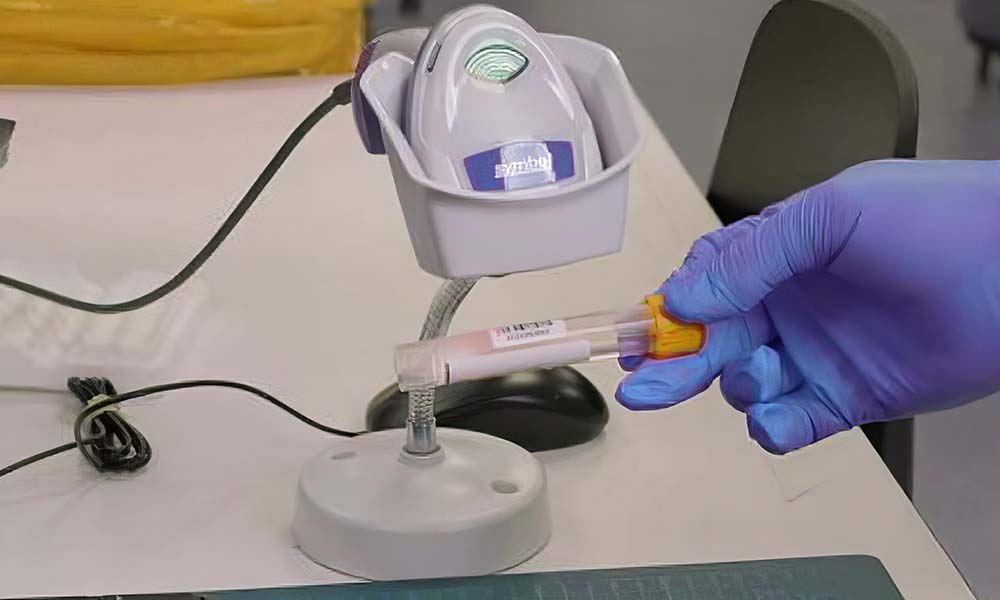
Benefits of Digital Accessioning
Automated Data Entry
Digital systems can automatically process sample information from barcodes or RFID tags as soon as a sample is received, significantly reducing the time required to process each sample. Automated data capture also reduces the risk of transcription errors, ensuring that sample information is accurately recorded2.
Streamlined Sample Tracking
Digital accessioning systems provide real-time tracking of samples throughout the laboratory workflow. Samples can be easily located using digital records, reducing the time spent searching for missing samples2,3. This is especially useful in high-throughput settings, where keeping track of a very large number of samples can be challenging4.
Enhanced Workflow Integration
Digital manifests seamlessly integrate with other laboratory information management systems (LIMS) and user platforms, facilitating the automatic transfer of sample data between systems. This integration streamlines the entire workflow, from sample receipt to analysis and reporting, further reducing processing time and enhancing overall efficiency.
Scalability and Flexibility
Unlike manual processes, digital systems are highly scalable, meaning they can easily handle increased sample volumes without a corresponding increase in workload. This scalability is especially important for labs that experience fluctuating demand or are required to scale up in line with project needs4.
Overcoming the Barriers to Adoption
While the benefits of digital accessioning are clear, some laboratories may be hesitant to make the switch due to concerns about cost or the need for new equipment and training. However, the long-term savings in time and resources often outweigh the initial investment. Additionally, many digital systems are designed to be user-friendly, minimizing the learning curve for staff. It is also essential to consider the potential for improved data integrity and compliance. Digital systems provide detailed audit trails, helping laboratories meet regulatory standards and maintain high levels of traceability. By reducing the risk of errors and improving data management, digital manifests not only save time but also enhance the overall reliability of laboratory operations.
Sampled’s Digital Accessioning Framework
At Sampled, we use a barcode-based sample accessioning system integrated with our core LIMS, LabVantage, and our proprietary system, SampledSphere, for customer access. Combining these digital components with robust quality control measures allows us to guarantee safe sample storage and efficient, reliable analytics to our customers in line with International Society for Biological and Environmental Repositories (ISBER) best practices and regulatory requirements6,7.
Conclusion
Digital accessioning systems offer a faster, more accurate, and scalable solution to the challenges of manual processes. By automating data entry, streamlining sample tracking, and integrating seamlessly with other laboratory systems, digital accessioning can significantly reduce the time and effort required to manage samples, allowing labs to focus on what truly matters – delivering accurate and timely results.
For labs looking to enhance efficiency and improve the reliability of their workflows, transitioning to digital accessioning is a smart move.
To learn more about the benefits of digital manifests and how they can transform your laboratory operations, download our full white paper on this topic.

References
1. Nakhleh RE, Idowu MO, Souers RJ, Meier FA, Bekeris LG. Mislabeling of Cases, Specimens, Blocks, and Slides: A College of American Pathologists Study of 136 Institutions. Arch Pathol Lab Med. 2011;135(8):969-974. doi:10.5858/2010-0726-CPR
2. Norgan AP, Simon KE, Feehan BA, et al. Radio-Frequency Identification Specimen Tracking to Improve Quality in Anatomic Pathology. Arch Pathol Lab Med. 2020;144(2):189-195. doi:10.5858/arpa.2019-0011-OA
3. Nakhleh RE. Lost, Mislabeled, and Unsuitable Surgical Pathology Specimens: Pathol Case Rev. 2003;8(3):98-102. doi:10.1097/01.PCR.0000065693.59517.7E
4. Pettit ME, Boswell SA, Qian J, Novak R, Springer M. Accessioning and automation compatible anterior nares swab design. J Virol Methods. 2021;294:114153. doi:10.1016/j.jviromet.2021.114153
5. Snyder SR, Favoretto AM, Derzon JH, et al. Effectiveness of barcoding for reducing patient specimen and laboratory testing identification errors: A Laboratory Medicine Best Practices systematic review and meta-analysis. Clin Biochem. 2012;45(13-14):988-998. doi:10.1016/j.clinbiochem.2012.06.019
6. ISBER. Best Practices: Recommendations for Repositories Fifth Edition. Published online 2023. Accessed January 29, 2024. https://cdn.ymaws.com/www.isber.org/resource/resmgr/best_practices/ISBERBestPractices-5thEditio.pdf
7. Health Insurance Portability and Accountability Act of 1996 (HIPAA) | CDC. June 28, 2022. Accessed March 4, 2024. https://www.cdc.gov/phlp/publications/topic/hipaa.html

