In a manuscript published in Developmental Cognitive Neuroscience, co-authored by Sampled Senior Director of Scientific Affairs, Dr. Michael Sheldon, researchers present the rationale and protocol for the HEALthy Brain and Child Development (HBCD) study.
This research involves collecting biospecimens from 7,000 birthing parents and their children to investigate how prenatal and postnatal experiences influence children’s neural, cognitive, behavioral, social, emotional, and health outcomes.
The Impact of Early Experiences and Exposures on Infant Health Outcomes
The development of the human nervous system is a complex process, beginning a few weeks after conception and continuing throughout gestation and long after birth. This development is driven by a complex interplay of genetic and environmental factors, with the late prenatal stage and early infancy marking a period of exceptionally rapid and significant change. Although it is well understood that these factors impact childhood development across biological and social domains, the precise causal relationships between early exposures and subsequent behavior remain unclear. Therefore, gathering diverse data from birthing parents and their children during both prenatal and postnatal stages is essential for uncovering the biological mechanisms that link early-life exposures to developmental outcomes.
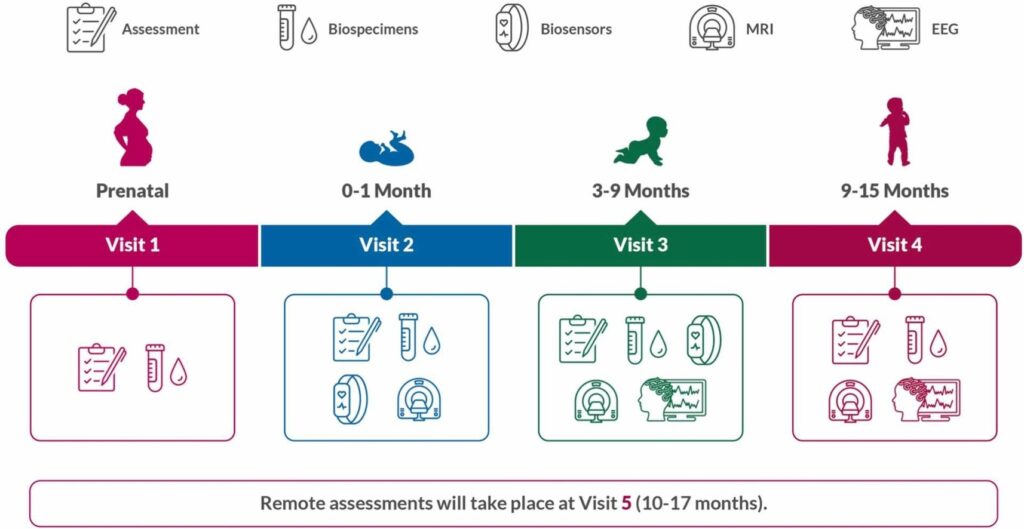
In this manuscript, the research team presents the rationale behind the study, detailing the selection of specific biospecimen collections (e.g., blood, saliva, nails) and other tests conducted at various developmental stages (i.e., prenatal, 0–1 month, 3–9 months, 9–15 months). The study design aims to balance the depth of phenotypic information collected with considerations of participant burden and other relevant factors.
EL Sullivan, R Bogdan, L Bakhireva, et al., Biospecimens in the HEALthy Brain and Child Development (HBCD) Study: Rationale and protocol, Developmental Cognitive Neuroscience, Volume 70, 2024, 101451, ISSN 1878-9293. https://doi.org/10.1016/j.dcn.2024.101451.
Cohort Selection
The HBCD study was designed to consider population diversity and specific substance exposures. Accurate representation of the broader population is essential to minimize bias in large cohort studies. At the same time, a key goal of the study is to examine infant exposure to substances such as opioids, marijuana, alcohol, tobacco, and others. To achieve both aims, the researchers organized the cohort into three categories.
- 50% – Racially, ethnically, and socioeconomically diverse cohort representative of the U.S. population
- 25% – Pregnant persons with use of targeted substances
- 25% – Individuals demographically and behaviorally similar to group two but without substance use during pregnancy
All participating birthing parents are between 18 and 50 years of age and will have given birth 12 months before the start of the study.
Parameter Selection
Data is collected from biospecimen analysis (i.e., genomics/epigenomics), questionnaires, interviews, biosensors, behavioral, electroencephalogram (EEG), and magnetic resonance imaging (MRI). This enables researchers to link prenatal and postnatal experiences, including:
- Physical health
- Behavior
- Neurocognition/language capabilities
- Neurodevelopment
- Activity and sleep
Biospecimen collection is a central component of the HBCD study, providing researchers with objective insights into various biological mechanisms and exposure to specific target substances. The types of biospecimens to be collected and the rationale for their collection include:
- Blood: To assess biomarkers and environmental exposures in the birthing parent
- Urine: To assess proteins and environmental exposures
- Nails: To index substance exposure
- Saliva: For genetic material and to track epigenetic changes over time
- Stool: To facilitate microbiome studies in child participants
Sampled, a CAP-accredited and CLIA-licensed analytical laboratory and biorepository, performs the ongoing specimen collection logistics, sample storage, and analytical processing.
Implications for Research
The HBCD study aims to generate a comprehensive collection of high-quality biospecimens and associated data for future use by diverse research teams. In addition to providing these resources, the researchers offer guidance on effectively applying the data to various fields, including genomics, nutrition, toxicology, inflammation, microbiome, and metabolomics. The depth and robustness of this data will support a wide range of research areas and help address longstanding questions about how early experiences and exposures influence neurocognitive and behavioral development.

“The design of the HBCD study, pairing the longitudinal collection of extensive phenotypic data with varied biospecimens, will enable researchers to interrogate the complex relationships in child development between genetics, environment and maternal drug exposure using state-of-the-art multiomics approaches including bulk and single cell next generation sequencing, epigenomics, metagenomics, and metabolomics.“
Michael Sheldon, PhD
Dr. Sheldon has a career spanning more than 30 years in genetic research and biobanking. In 2021, he founded the Scientific Affairs department at Sampled with the mission to provide expert technical resources to clients, discovery and adoption of new technologies, and the coordination of outreach initiatives. Prior to that he served as Senior Director of Sample Processing Services at RUCDR Infinite Biologics (now Sampled), with oversight of all sample processing services relating to blood fractionation, cell and stem cell culture, and nucleic acid extraction.
Dr. Sheldon received his B.A. from Cornell University in 1983 and a Ph.D. from SUNY at Stony Brook in 1993. He is an Adjunct Professor of Genetics at Rutgers University and is a published researcher in Genetics, Neurodevelopment, and Stem Cell biology.

