The recent webinar “Long-read Whole Genome Sequencing: Enhancing Diagnostic Power Across Clinical Applications“, hosted by Genetic Engineering & Biotechnology News (GEN), saw Shareef A. Nahas, PhD, Chief Scientific Officer at Sampled, and Katarzyna (Kasia) Ellsworth, PhD, Senior Director of Clinical Operations at Rady Children’s Institute for Genomic Medicine (RCIGM), sit down to discuss and present data on the emergence of long-read sequencing as a practical, high-impact technology for improving diagnostic yield in clinical genomics workflows and beyond.
The webinar delivered data-driven insights into the current state of long-read sequencing, with a focus on clinical practice, presented from the perspectives of both a clinician and a medical genomics expert. It is essential viewing for clinical operations managers and organizations seeking to improve diagnostic yield and ensure that their strategies for 2026 are aligned with newer technologies that are cost-effective, practical, and make a meaningful contribution to patient care.
The Importance of Clinical Genomics, and the Missing Pieces
Genetic variation is the root cause of many diseases. While many variants are well characterized and their clinical implications are understood, the genetic causes of many rare and complex conditions remain unknown or only partially elucidated. Single-nucleotide mutations and other small variants play an important role in genetic disease, but they do not tell the full story. Larger variants, including structural variants and repeat expansions, are known contributors to disease, but their full impact on the genetic disease landscape remains poorly understood. Short-read sequencing is highly effective for detecting small genetic variants, but it struggles to resolve larger variants, leaving many patients without a definitive diagnosis and clinicians with limited information with which to guide treatment.
Long-Read Sequencing Comes of Age
Dr. Nahas opened the discussion with an overview of current long-read sequencing technologies, highlighting how the field has undergone a dramatic transformation in recent years. Historically, long-read sequencing has been low-throughput and expensive, and used only for niche applications and small cohort studies. Today, it offers much higher throughput at a lower cost, making it suitable for clinical and commercial applications.
Dr. Nahas explained how long-read sequencing provides accurate detection of different variant classes to support rare and complex disease diagnosis. It is also valuable in pharmacogenomics, where it enables accurate star-allele resolution, and in population genomics, where it enables high-accuracy phasing. Long-read sequencing can significantly improve clinical interpretation and epidemiological insights, especially for populations underrepresented in existing genomics studies. It also excels in preclinical GLP gene-editing assessments, where it helps evaluate clonal stability, detect unintended edits, and generate high-quality reports for regulatory review. In short, it is a powerhouse across commercial, clinical, and translational pipelines, and is already in use by early adopters.
Dr. Nahas also presented a practical framework for implementing long-read whole-genome sequencing (WGS) within CLIA/CAP-regulated clinical environments, including detailed guidance on the analytical validation of short tandem repeat coverage across diverse loci. This section of the webinar is a must-watch for any organization considering adopting long-read WGS into their clinical workflows.
A Clinician’s Perspective on Long-Read Sequencing
In the second part of the webinar, Dr. Ellsworth provided her perspective on long-read sequencing as a clinician working with patients with genetic disorders. Dr. Ellsworth began by highlighting the power of short-read sequencing in rapid clinical genetic testing. She then outlined the limitations of short-read sequencing, including alignment ambiguity caused by high sequence similarity, reduced sensitivity to larger variants, and difficulties detecting repeat expansions and epigenetic features that are critical for the analysis of many neurological disorders.
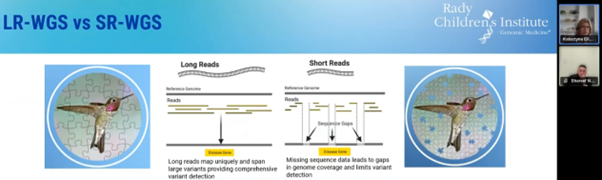
Dr. Ellsworth presented several practical examples illustrating the contrasting ability of long-read and short-read sequencing to resolve short tandem repeats in clinical samples. Some short-read sequencing reads spanning repeat regions were lower in quality, leading to less reliable variant calls, whereas long-read sequencing provided improved resolution in these regions. Based on these findings, the team at RCIGM recognized the value of incorporating long-read sequencing to improve diagnostic yield and reduce final report turnaround time in certain cases. Dr. Ellsworth and her team now include long-read sequencing as an integrated part of their WGS pipelines for repeat expansion detection.
Making Large-Scale Long-Read Sequencing a Reality
Dr. Nahas and Dr. Ellsworth agreed that the question is no longer whether long-read sequencing belongs in clinical diagnostics, but how it can be integrated without disrupting existing operations. As costs decrease and throughput increases, the application of long-read sequencing is likely to expand significantly in the clinical setting. Laboratories that invest early and maintain flexible sequencing and downstream analysis infrastructure will be best positioned to benefit.
To conclude the webinar, Dr. Nahas and Dr. Ellsworth engaged in an insightful discussion on what it truly takes to implement long-read sequencing at scale, covering critical considerations such as DNA quality and quantity, extraction methods, and the need for purpose-built facilities. Their conversation reinforced that success depends not only on technology, but on robust, CLIA/CAP-compliant frameworks and expert-led processes that integrate seamlessly into existing clinical genomics programs.
Watch the full webinar on-demand now
Ready to try long-read sequencing for yourself?
Sampled is a Certified Service Provider (CSP) for the PacBio Revio and offers end-to-end long-read sequencing services.
Contact our team to see how you can use long-read sequencing to unlock genomic insights and achieve improved outcomes in your clinical or commercial workflows.


