Functional Quality Control: What is it and why do we do it?
This blog aims to answer these two questions to introduce our Functional Quality Control (FQC) testing performed in our Global Integrated Analytical Biorepository (GIAB) to assess sample quality and validate subject identity.
What is FQC Testing?
In order to mitigate unforeseen errors during sample collection, transport and processing we perform FQC testing. An FQC test involves running a custom Genotyping SNP assay on every sample that is processed in our laboratory or enters our GIAB, in which DNA extraction is performed – this provides a range of benefits including:
- Our FQC assay serves as a quality check that provides confidence in the performance of the sample in downstream applications. This ensures that clients are not running assays with a high likelihood of failure – saving time and money.
- DNA fingerprinting enhances sample tracking as they undergo multiple processing steps – an added layer of security for potentially irreplaceable samples.
- Provides a concordance test to verify sample authenticity and manually collected patient phenotype.
- Verification that sample is from the same patient with longitudinal studies.
- Provides backstop for review if clients still want to run the sample – enabling a greater understanding through more data points.
- Helps to flag any external issues with collection, transportation, or handling – supporting standardization across large scale trials with multiple collection sites.
Essentially, FQC is the process by which we ensure that samples and data generated from their analysis are of a high enough standard to assure reliable and repeatable results with added benefits of security and sample damaging event flagging.
Why perform FQC Testing?
There are several reasons that make us advocate FQC testing to our clients, these include valid assays, financial viability, rich datasets, and sample identification:
Valid Assays
The primary purpose of running an assay is to gain insights from the results. The quality of the insight gained therefore is directly related to the assay being valid and providing reliable, accurate and reproducible results. Running our FQC test ensures that the samples being run in concordant tests are to the correct standard required for the assay to be valid.
Financial Viability
Many assays can be expensive to run, and long-term storage cost for future testing can also require a continued investment. Running an FQC test to inform how successful the assays are likely to be or whether the sample is worth storing can protect our clients’ investments and make the most of their budgets or research grants.
Rich Datasets
Despite FQC results, many clients opt to run samples that may have failed the FQC test anyway to ensure they are maximizing their opportunity during clinical trials. Samples that have failed during the assays can then be checked against the FQC data to determine if those particular results can be discounted from research. This provides rich datasets for researchers to draw upon during analysis.
Sample Identification
Running FQC gives us a DNA fingerprint (similar to forensic testing) of each sample, enabling an added layer of security in sample tracking. Unless clients opt for an alternative custom solution, we operate a standard of uniquely barcoded sample storage tubes and racks that are recorded against the sample in our LIMS platform – having a DNA fingerprint provides a redundant back up of the sample record that we can run a profile match against in our database.
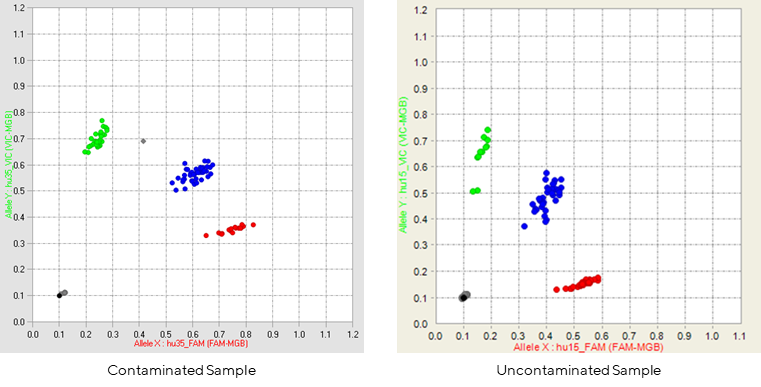
Figure 1: Contaminated sample vs. uncontaminated sample.
Scatter Plot Key
The images above are typical scatter plot, each dot represents the genotype for one sample on that SNP.
Samples will typically cluster into 4 regions, indicative of the genotype at that locus (HOM= homozygous, HET = heterozygous): No Call (black), HOM A (green), HOM B (red), or HET (blue), enabling gender concordance to be established.
The image on the left is an example of a profile that was observed with a possible contamination, the grey dot between the green and blue clusters, clustering for the remaining SNPs for that sample would need to be checked to verify the status of that sample.
A failed run will not show these 4 clear clustering patterns.
Summary
FQC testing is performed to ensure reliable and repeatable valid assays through pre-qualification of samples ahead of more extensive and expensive testing. It also serves a number of other functions such as financial viability, rich datasets and sample identification.
The benefits of FQC testing assure that clients are getting not just high-quality research insights and security, but they are also able to maximize their budgets and grants through confidence in valid assays and future utilization of the samples after storage.
Learn more about how we can support your research whilst ensuring the potential of your precious samples is maximized, contact one of our experts today.

